- MLN Matters Number: SE20011 ... Medicare Fee-For-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19) was revised and posted November 9, 2020. This is a 19-page MLN Matters.

Pages 14 through 16 speak to clarification of SNF billing instructions as noted above. You’ll easily spot the red ink on those pages. Page 13 begins the section on Skilled Nursing Facility (SNF) Benefit Period Waiver - Provider Information.
- CDC updated its Healthcare Facilities That Have Implemented COVID-19 Electronic Case Reporting website on November 9, 2020.
- The CDC Guidance for SARS-CoV-2 Point-of-Care Testing on November 9, 2020. This CDC Web resource provides guidance on the regulatory requirements for SARS-CoV-2 POC testing, using POC tests safely, and information on reporting POC test results. POC tests are used to diagnose COVID-19 in various settings, such as:
- Physician offices
- Urgent care facilities
- Pharmacies
- School health clinics
- Long-term care facilities and nursing homes
- Temporary locations, such as drive-through sites managed by local organizations
- The CDC 10 Things Healthcare Professionals Need to Know about U.S. COVID-19 Vaccination Plans website was also updated on November 9, 2020. With the possibility of one or more COVID-19 vaccines becoming available before the end of the year, these are 10 things healthcare professionals need to know about where those plans currently stand.
A related website How CDC Is Making COVID-19 Vaccine Recommendations was also updated that same day.
- QSO-21-04-CLIA ... Clinical Laboratory Improvement Amendments of 1988 (CLIA) CMS Locations and State Agency Remote Survey Guidance - Optional Process was posted November 6, 2020.
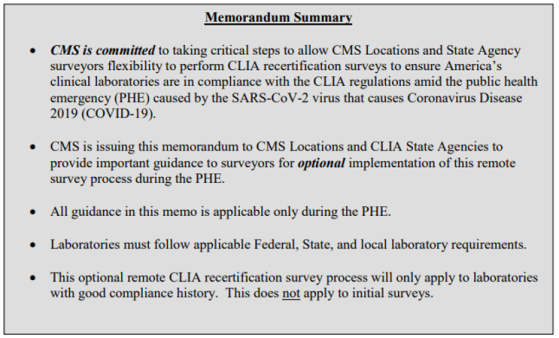
This relates to laboratories being surveyed. The 5-page QSO includes 2 pages of CLIA Remote Survey Process - Surveyor Use Only steps.
- Lessons Learned: Frequently Cited Standards Related to COVID-19 Inspections is an interesting read posted late last week.

The hyperlinks (in blue) will take you to additional information/resources.
Want to keep up with the changing COVID-19 situation in skilled nursing?




