- MLN Matters Number: SE20011 Revised was posted on Friday, July 17, 2020.

This is a 16-page MLN. Look for the red ink within this document. The first occurrence is found on page 11.

Keep reading as there is more clarification regarding the SNF Benefit Period Waiver as well as billing.
- Testing Guidelines for Nursing Homes were updated by CDC on Friday, July 17, 2020.

- Strategies to Mitigate Healthcare Personnel Staffing Shortages was also reviewed and updated by CDC on Friday, July 17, 2020. There is no Summary of Changes for this posting.
- Interim Guidance on Testing Healthcare Personnel for SARS-CoV-2 was also reviewed and updated by CDC on Friday, July 17, 2020. There is no Summary of Changes for this posting.
- Clinical Questions about COVID-19: Questions and Answers was reviewed and updated by CDC on Friday, July 17, 2020; also, no Summary of Changes provided. I’ve blogged on this resource before – it’s a compilation of Q&A in drop-down format.
- Criteria for Return to Work for Healthcare Personnel with SARS-CoV-2 Infection (Interim Guidance) was updated by CDC on Thursday, July 16, 2020 and posted July 17, 2020.

- Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19 was also updated by CDC on Friday, July 17, 2020.

- Overview of Testing for SARS-CoV-2 was updated by CDC on Friday, July 17, 2020.

- The July 2020 issue of JAMDA (Journal of Post-Acute and Long-Term Care Medicine) contains these 3 interesting articles:
- Solving the COVID-19 Crisis in Post-Acute and Long-Term Care … “This special article presents 5 keys to solving the COVID-19 crisis in post-acute and long-term care, related to policy, collaboration, individualization, leadership, and reorganization. Taking action during this crisis may prevent sinking back into the complacency and habits of our pre-COVID-19 lives.” This is the link to the 3-page PDF of the article.
- Policy Recommendations Regarding Skilled Nursing Facility Management of Coronavirus 19 (COVID-19): Lessons from New York State … “To provide policy recommendations for managing Coronavirus 19 (COVID-19) in skilled nursing facilities, a group of certified medical directors from several facilities in New York state with experience managing the disease used e-mail, phone, and video conferencing to develop consensus recommendations. The resulting document provides recommendations on screening, protection of staff, screening of residents, management of Coronavirus 19 positive and presumed positive cases, communication during an outbreak, management of admissions and readmissions, and providing emotional support for staff. These consensus guidelines have been endorsed by the Executive Board of the New York Medical Directors Association and the Board of the Metropolitan Area Geriatrics Society.”
- Practical Steps to Improve Air Flow in Long-Term Care Resident Rooms to Reduce COVID-19 Infection Risk … “This article outlines 5 pragmatic steps that long-term care facilities can take to manage airflow within resident rooms to reduce the potential for spread of infectious airborne droplets into surrounding areas, including hallways and adjacent rooms, using strategies adapted from negative-pressure isolation rooms in acute care facilities.”
- COVID-19 Testing in Long-Term Care Facilities is a website that you’ll want to check out and bookmark for future reference. There are several components on this page. Here’s the remarks found in the Introduction: “Many states are prioritizing and, in some cases, requiring COVID-19 testing of all residents and staff at long-term care (LTC) facilities. This brief provides considerations for developing a strategy for COVID-19 testing in LTC facilities and a review of state actions to date. Additional details on testing and other state strategies for LTC facilities, including specific state resources can be found here. For the purposes of this brief, LTC facilities include nursing facilities (including post-acute), assisted living, and other residential care settings and testing approaches may vary across these settings.” The 2 hyperlinks were most recently updated on July 8, 2020. The snippet below shows what can be found beneath the 2nd hyperlink (the here above):
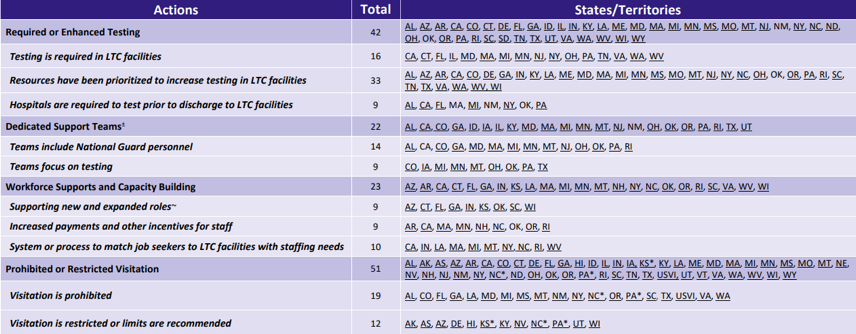

It complements the 1st hyperlink provided in this bullet which shows:

- Trump Administration Announces Initiative for More and Faster COVID-19 Testing in Nursing Homes is the press release posted July 14, 2020 announcing the HHS “This initiative is a one-time procurement of devices and tests targeted to facilitate on-site testing among nursing home residents and staff. Through this crucial action, nursing homes will be able to augment their current capacity for coronavirus testing, bolstering their response and helping to prevent the spread of SARS-CoV-2, the virus that causes COVID-19.”
“Distribution will begin next week with nursing homes prioritized by Centers for Medicare & Medicaid Services (CMS) in their continuing effort to protect older adults. Each nursing home will receive one diagnostic test instrument and associated tests. Following initial distribution, nursing homes can procure additional tests directly from the respective manufacturers. Nursing homes must have the capability to screen and test residents, and test staff on a weekly basis or according to specific guidance by the state and local health departments. This procurement will also enable testing of visitors if appropriate for that facility.”
Operators Greet NH COVID Testing Initiative with Cautious Optimism provides a view of this initiative from AMDA perspectives.
- Toolkit on State Actions to Mitigate COVID-19 Prevalence in Nursing Homes … Version 5 has been posted. This version comes within 3 weeks of the Version 4 Toolkit and is now 151 pages in length.
- COVID-19 Frequently Asked Questions (FAQs) on Medicare Fee-for-Service (FFS) Billing was most recently updated on Wednesday, July 15, 2020. Updated FAQs include:
V. Cost Reporting
1. Question: Will CMS delay the filing deadline for cost reports impacted during the COVID-19 PHE?
Answer: Yes, 42 CFR 413.24 (f)(2)(ii) allows this flexibility. CMS will delay the filing deadline of Fiscal Year End (FYE) 10/31/2019 and FYE 11/30/2019 cost reports until June 30, 2020. CMS will also delay the filing deadline of the FYE 12/31/2019 cost reports until August 31, 2020. For the FYE 01/31/2020 cost report, the extended due date is August 31, 2020. For the FYE 02/29/2020 cost report, the extended due date is September 30, 2020. In summary the extension impacts the following cost reporting fiscal year ends for all provider types (hospitals, SNFs, HHAs, hospices, ESRDs, RHCs, FQHCs, CMHCs, OPOs, histocompatibility labs and home office cost statements): Revised: 7/15/20
DD. Medicare Payment to Facilities Accepting Government Resources
1. Question: Can a skilled nursing facility (SNF) or hospital accept Federal, State, or local government resources (e.g., supplies and staffing assistance) to help with the COVID-19 emergency and still bill Medicare?
Answer: Yes. Although Medicare usually doesn’t allow payment for services that are paid for by a governmental entity, there is an exception for services furnished as a means of controlling infectious diseases (see 42 CFR 411.8(b)(4)). Updated: 7/15/20
Want to keep up with the changing COVID-19 situation in skilled nursing?




