On December 22, 2020, CMS unveiled its list of quality and efficiency measures under consideration – a precursor or first step in the pre-rulemaking process. In that Press Release, CMS revealed the 2020 list, which includes a number of new measures, as well as several updates to modernize or replace existing measures:
- Five outcome measures (measures that focus on the results of health care provided through Medicare), such as the rate of health care-associated infections requiring hospitalization for residents of skilled nursing facilities;
- Five process measures (measures that emphasize efforts to promote standardized best practices), such as conducting kidney health evaluations or implementing interventions for patients with pre-diabetes (the medical term for blood glucose levels that are high but not yet high enough for a type-2 diabetes diagnosis). Importantly, the 2020 list includes three process measures for the coronavirus disease 2019 (COVID-19) vaccine. The measures under consideration list proposes looking at:
- Vaccination coverage among health care personnel,
- Vaccination by clinicians, and
- Vaccination coverage for patients in End-Stage Renal Disease (ESRD) facilities;
- Five cost/resource use measures (measures that evaluate how frequently health care items or services may be used, as well as how much they might cost) – including, for example, episode-based costs associated with addressing diabetes or asthma/chronic obstructive pulmonary disease;
- Three composite measures (which summarize overall quality of care across multiple measures through the use of one value or piece of information); and
- Two patient reported outcomes measures (measures where the information comes directly from the patient).
All but three measures under consideration rely on digital rather than traditional “pen-and-paper” data collection. Of the non-digital measures, two are measures aimed at assessing COVID-19 vaccinations among health care personnel and patients in ESRD facilities, and the other reflects key patient-reported health outcomes, which help prioritize patient voices and empower patients to take an active role in their health.
Every year, CMS evaluates all measures in its programs, proposing to remove those that have become less relevant and proposing new measures that may be more meaningful based on review by external health care experts. This year, almost all of the measures proposed would be collected digitally, meaning information comes from claims and other electronic sources, and would not require doctors to retrieve data manually. As a signal for CMS’s broader direction as the agency puts patients over paperwork in the push for quality and innovation, the 2020 list of measures under consideration represents “a first” on several important fronts, particularly where digital innovation and reducing administrative burden are concerned.
CMS expects to receive the MAP’s (Measure Applications Partnership) input on the 2020 measures under consideration by February 1, 2021. Experts at CMS and the Department of Health and Human Services will work collaboratively based on this assessment to select final measures available for further public comment through a notice of proposed rulemaking in the Federal Register.
For more information or to review the 2020 list of measures under consideration, please visit: https://www.cms.gov/files/document/measures-under-consideration-list-2020-report.pdf. This is an 85-page document that provides detailed information including this table by program:
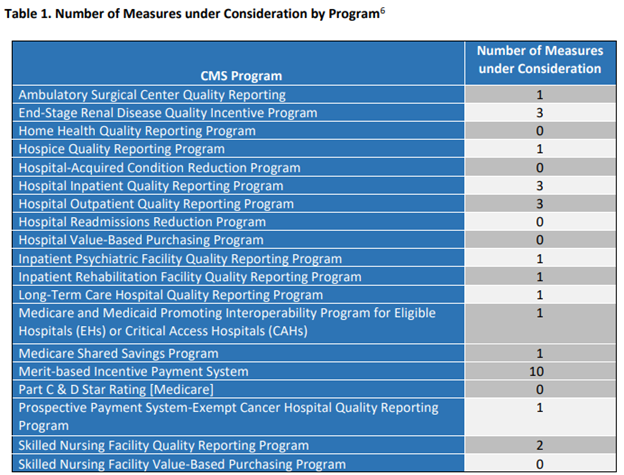
This document is well-worth the read ahead of expected rulemaking. I’ll be keeping an eye on this as well and will post updates as they become available.
Want to keep up with the changing COVID-19 situation in skilled nursing?



