The Department of Health and Human Services, Office of the Assistant Secretary for Health (OASH), recently announced that we will begin providing nursing homes with a Point of Care (POC) rapid response testing instrument to bolster each facility’s ability to prevent the spread of COVID-19. The data collected through the NHSN system directly supports this initiative by helping to prioritize the nursing homes with testing needs and an increasing number of cases. For the methodology describing how facilities are prioritized, and a listing of the facilities, please click here. A list of frequently asked questions (FAQs) is also available here. For more information about the testing instruments, please view our recorded webinar. Also, view our toolkit for nursing homes using point of care devices for SARS-CoV-2 testing as a quick reference guide to important information about testing.
- The Testing Toolkit is 9-pages in length. Here’s the TOC:

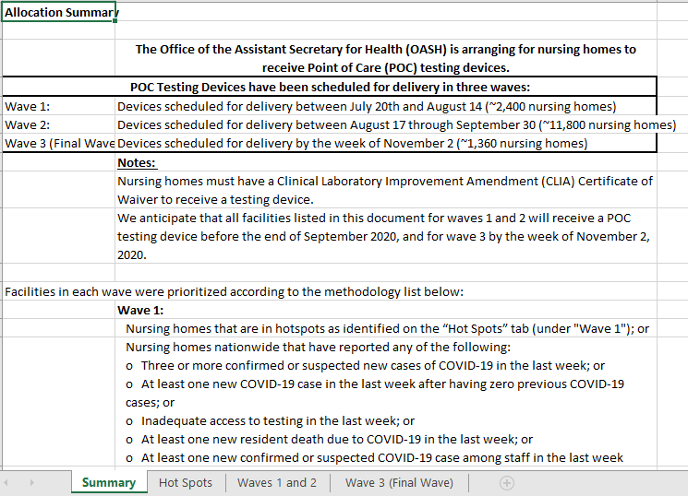
- The list of facilities (1st hyperlink identified as here above in introductory paragraph) is an Excel spreadsheet dated October 19, 2020. Note the tabs/pages at the bottom of the spreadsheet.
- The FAQ document (2nd hyperlink identified as here in introductory paragraph) is a 6-page document. There is no date on this document.
- The recorded webinar (CMS YouTube Channel) is 1 hour and 23 minutes in length and the link is designated as such in the introductory paragraph.

The purpose of this webinar is to assist states in their continued COVID-19 outreach with nursing homes, providing training about testing instruments and the nursing home rules testing mandate.
NHSN Long-Term Care COVID-19 Module — CDC’s National Healthcare Safety Network (NHSN) added a Point of Care Laboratory Reporting Pathway within the NHSN Long-Term Care COVID-19 Module. This added capability enables CMS-certified long-term care facilities to meet the Department of Health and Human Services’ requirement to report SARS-CoV-2 point-of-care antigen test data, and other on-site COVID-19 laboratory testing data.
The new NHSN pathway creates a single, standardized reporting system:
- that all ~15,400 nursing homes already use for other mandatory COVID-19 reporting;
- has the capability to share data with state and local health departments;
- has the ability to share data with HHS and CMS; and
- avoids the creation of a patchwork of different jurisdictional reporting systems by state health departments.
In order to utilize the new pathway to fulfill reporting requirements, nursing homes and other long-term care facilities that are NHSN users will need to upgrade their NHSN Secure Access Management Service (SAMS) from Level 1 to Level 3. CDC is working closely with facilities to assist them in this process. An email invitation from CDC to perform this upgrade will be sent to users. Alternatively, facilities can email nhsn@cdc.gov with the subject line “Enhancing Data Security” to begin upgrading their SAMS access to use this Pathway.
Guidance for SARS-CoV-2 Point-of-Care Testing — Point-of-care (POC) tests, such as some rapid tests for diagnosing an infectious disease, provide results within minutes of the test being administered, allowing for rapid decisions about patient care. POC tests can also extend testing to communities and populations that cannot readily access care. This page provides detailed information on the following:
- Overview of POC testing
- How to obtain a Clinical Laboratory Improvement Amendments (CLIA) certificate
- How to safely perform POC specimen collection, handling, and testing for COVID-19
- How to comply with result reporting requirements
To learn more, please visit: Guidance for SARS-CoV-2 Point-of-Care Testing
The CDC is hosting two identical training webinars on October 22 and 23 to introduce this new module. Both sessions will feature a live Q&A. A recording of the original webinar will also be posted for online viewing.
Registration details are below:
Webinar #1: Reporting Results of Point of Care Testing for COVID-19: A New NHSN Pathway
- Date: October 22, 2020
- Time: 11:00 AM – 12:00 PM ET
Webinar #2: Reporting Results of Point of Care Testing for COVID-19: A New NHSN Pathway
- Date: October 23, 2020
- Time: 2:00 – 3:00 PM ET
*Note this will be a re-broadcast of webinar #1 with a live Q&A Important note: Reporting through this module requires updating your Secure Access Management Services (SAMS) access to Level-3. Instructions on updating your access are available here.




